Welcome To Off The Radar
DR SIN HANG LEE - A CASE STUDY IN ETHICS DON'T PAY
- Hits: 9297
For Immediate Release
Contact: Norma Erickson, President
This email address is being protected from spambots. You need JavaScript enabled to view it.
October 4, 2011
By Norma Erickson, President
SANE Vax Inc. was organized to provide medical consumers with all of the information they need to make informed choices about vaccines, HPV vaccines in particular. Dr. Sin Hang Lee, Director of Milford Medical Laboratory Inc. has been instrumental in our efforts to raise consumer awareness regarding safe and effective cervical cancer prevention and the scientific facts in relation to the international HPV vaccine controversy.
Because of studies submitted to the FDA prior to approval of HPV vaccines indicating there was a substantial increase in pre-cancerous lesions for those who had already been exposed to vaccine-relevant genotypes of HPV before they were injected with Gardasil® or Cervarix®, Dr. Lee agreed to offer his cutting edge technology HPV genotyping test to medical consumers throughout the United States to protect those who were considering HPV vaccination so they could avoid that potential risk. The decision was announced to the public via a BusinessWire Press Release on 20 September 2010.
In the fall of 2010, without Dr. Lee’s knowledge or having an opportunity to defend himself, the newly appointed Chairperson of the Pathology Department at Milford Hospital informed the hospital’s credentialing committee that she was not recommending for approval or supporting Dr. Lee’s application for renewal of his medical staff privileges. For those who do not know, medical staff privileges at a hospital are a major asset to a medical doctor and they establish the relationship that permits among other things, the doctor to practice at a particular hospital. When medical staff privileges at a hospital are revoked or not renewed, the doctor no longer has permission to practice at the hospital or use its facilities. The non-renewal of the medical staff privileges, may also adversely affect the doctor’s license to practice medicine. In Dr. Lee’s case, the non-renewal of his medical staff privileges at Milford Hospital is under appeal.
Although Dr. Lee still maintains his medical staff privileges during the appeal, his position as director of the laboratory was summarily terminated along with his employment relationship at Milford Hospital on December 13, 2010 and he has been prevented from using the hospital’s laboratory to continue his testing and research there ever since that time. A lawsuit addressing the wrongful termination claim has been brought against the Milford Hospital.
While the hospital-based appeal hearings and the lawsuit are pending, Dr. Lee’s research and testing and the operation of his world class, CLIA certified molecular diagnostic laboratory at the Milford Hospital have all been significantly hindered and obstructed. Dozens of opportunities for Dr. Lee to test Gardasil samples for contaminants have been lost as the hospital has redirected and/or returned vaccine lots sent to and intended for Dr. Lee back to the senders or other locations. Dr. Lee and his attorneys are pursuing all available legal remedies to restore the testing laboratory as soon as possible in order to protect the public health, safety and well being.
TRUTH ABOUT GARDASIL - CYNTHIA JANAK
- Hits: 16651
September 27, 2011
MERCK has made public their "exclusion" criteria for the Gardasil HPV vaccine in documents filed with ClinicalTrials.gov, for clinical trial #NCT01096134, a.k.a. "Mother Daughter Initiative." If these "exclusion criteria" were known by, and applied to families in the United States of America, prior to the vaccination of their child, virtually none of the 22,000 girls and boys listed by the CDC's VAERS reporting system as being injured by the Gardasil HPV vaccine, would have been allowed to be vaccinated, and 100 deceased HPV vaccinated children, would still be alive today. Family medical histories of children injured by the Gardasil HPV vaccine have been compiled by the non-profit group, "TRUTH ABOUT GARDASIL," who has issued this statement.
After months and months of intensive research Truth About Gardasil has discovered that Merck the manufacturer of HPV vaccines have the potential to cause harm to people with certain pre-existing conditions that are not mentioned in the Physicians Product Insert (1)
In the clinical studies (2, 3, 4, 5, 6) we have found that certain individuals have been excluded from the clinical study groups. They are:
- Allergies to any component of the vaccine
- History of a severe allergic reaction
- Known history of any allergies to food or medicine
- Immunocompromised, Immunodeficient or has an autoimmune condition
- History of any condition, therapy, lab abnormality or other circumstance such that it is not in the best interest of the participant to participate
- Clinically significant disease or clinically significant findings during the screening medical history or physical examination that, in the investigator's opinion, would compromise the outcome of this study.
- Have a weakened immune system or other immune problems
The Gardasil® vaccine produced by Merck contains AAHS (amorphous aluminum hydroxyphosphate sulfate) also termed MAA (Merck Aluminum Adjuvant) (7, 8, 42) There is no effort at disclosure of the sulfate in the product under item 4. Contraindications of the Physicians Package Insert. (1) People with an allergy to any type of sulfate have the potential to be at risk for an adverse event that could result in anaphylactic response which is life threatening. (7)
DON'T GIVE GARDASIL TO YOUR DAUGHTER - DR MERCOLA
- Hits: 12756
Don't Give This to Your Daughter – Despite What Your Doctor Says
by Joseph Mercola
It's been four years since Gardasil debuted as a blockbuster vaccine with sales that rocketed to over $1.1 billion in its first nine months.
Touted as a wonder vaccine that would end cervical cancer, it was supposed to be the savior of both mankind and Merck's Vioxx-damaged bottom line. But now, according to CNN Money, it's a dud.
It just posted $219 million in sales. But in the pharma world, that's a paltry pittance, nothing short of an in-flight explosion that's caused Merck stock to drop 3 percent, with analysts and investors scrambling to figure out what went wrong.
So what happened?
How did a vaccine that was supposed to be Merck's beacon for higher profits in the 21st Century go from flagship to flop?
The Science Speaks for Itself
CNN Money calls Gardasil's crash a "design flaw" and faults the economy, puritanical parents, bad press, and Merck itself for contributing to the fallout.
The article ends with the hypothesis: "Or, maybe people just aren't ready for a cancer vaccine when it's for a sexually transmitted disease."
I think they're way off the mark.
The real reason Gardasil is a flop is that people have become educated about this vaccine.
They've looked at the science and weighed the risks vs. the supposed benefits, and have made a choice not to get it for themselves or their children.
The word is out: despite what the CDC would have you believe, Gardasil's safety record is in serious question. As of September 28, 2010, the Vaccine Adverse Events Reporting System (VAERS) has more than 18,000 Gardasil-related adverse events listed in it, including at least 65 deaths.
As a vaccine used in the developed world, the science speaks for itself: Gardasil can't – and never will – replace Pap smears, which are the reason that the incidence of cervical cancer is so low in the United States after decades of including pap smears in routine medical care for women.
Today, cervical cancer is not even in the top 10 cancers that kill American women every year.
As a vaccine for children, it doesn't make sense to vaccinate to try to prevent an infection that is cleared from your body without any negative effects within two years in most healthy persons, and is not transmitted in a school setting like other airborne diseases that are easily transmitted in crowded conditions.
GENETICALLY ENGINEERED HPV DNA IN GARDASIL
- Hits: 11089
FDA questioned about genetically engineered HPV DNA in Gardasil worldwide.
All New Zealand samples contaminated.
A few days ago, an American organisation put on their website a copy of a letter sent to the FDA, in which they stated that thirteen samples of Gardasil from Poland, France, Spain, USA, New Zealand and Australia, made in four different Merck factories (USA, Holland, France and CSL Australia) had been tested, and found to contain genetically engineered dna fragments which the purification process failed to remove. Yesterday, a more detailed press release was put onto SANE-Vax's site, which showed samples contaminated with HPV DNA 11 and 18. The New Zealand results can be seen here.One of the genetically engineered particles found is said to be a genetically engineered syntheticaly constructed gene designed to instruct the yeast or baculovirus cells, to make the HPV-11 outer capsid protein (virus-like particles) for the Gardasil vaccine.
This is the Pubmed site for GenBank Locus SCU55993, which is the picture below, of ONE of the alleged contaminants:
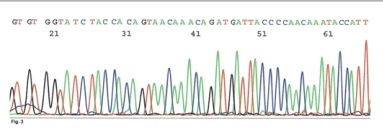
Wavy lines mean nothing to me, and probably nothing to you.
SANE-vax's letter doesn't specifically say what the other contaminants are, but say that the rest of their proof is available for review provided that FDA provides " appropriate safeguards ... to protect the proprietary processes and information utilized by our laboratory to test the samples."
To me, no platitudes and fob-offs on this issue are acceptable.
The first question that comes to mind is,
"Is this a big deal?"
This isn't a quality issue in one factory.
The problem, according to the testing done by SANE-vax shows;
A global manufacturing fault.
A global manufacturing issue relating to vaccine design, process and purity - IS - a big deal.
The next question is:
Are the "genetically altered HPV DNA fragments" a big deal?
I don't know, since this is going where no-one has gone before.
This is not like the situation recently when porcine viruses were found, as a contaminant of tripsin in the Rotavirus vaccine.
We are talking about the alleged laboratory identification of ONE specific genetically modified viral sequence (GenBank Locus SCU55993) spliced into the DNA of another species, for the manufacture of Gardasil, plus other so far unspecified "contaminants".
"Do the manufacturers of Gardasil claim there is no DNA in Gardasil?"
Yes they do!
During the licensure process Merck assured the FDA that Gardasil had no DNA in it, but nowhere on the FDA website are there any tests which proved that. And this is the first ever vaccine which specifically claims there is no DNA in the vaccine.
As a matter of historical interest, Dr Maurice Hilleman, whose name is synonymous with Merck said in this article about mammalian cell cultures:
"The principal concern for safety lies with retention of residual DNA in the vaccine, especially since induction of cancer is a single-cell phenomenon, and a single functional unit of foreign DNA integrated into the host cell genome might serve to induce cell transformation as a single event or part of a series of multifactorial events. Current proposed standards for vaccines would permit contamination with up to 100 pg of heterologous DNA per dose. This is equivalent to about 10(8) "functional lengths" of DNA. Total safety would seem to require complete absence of DNA from the product."
GARDASIL contamination with HPV Recombinant DNA
- Hits: 11919
By Leslie Carol Botha, Vice President of Public Relations
This email address is being protected from spambots. You need JavaScript enabled to view it.
September 5, 2011
SANE Vax Inc. contracted with an independent lab to test for contamination and found HPV recombinant DNA (rDNA) in 13 vaccine vials. The Gardasil vials with different lot numbers were from New Zealand, Australia, Spain, Poland, France and three states in the U.S. 100% of the samples tested positive for the presence of the genetically modified HPV DNA.
Dr. Sin Hang Lee, a pathologist at the Milford Hospital pathology laboratory well-known for using cutting-edge DNA sequencing for molecular diagnoses, was initially contracted to examine a single sample of Gardasil for possible contamination. This sample tested positive for recombinant HPV-11 and HPV-18 residues, both of which were firmly attached to the aluminum adjuvant.
In a certified letter mailed to FDA Commissioner, Dr. Margaret Hamburg on August 29, 2011, SANE Vax Inc. requested ‘the FDA investigate the extent of the HPV DNA contamination in the Gardasil HPV4 vaccine currently on the market and take appropriate actions to ensure public safety regarding future shipments.’ 1.
Why Did SANE Vax Inc. Investigate Possible Gardasil Contamination?
The mother of a sexually naïve adolescent girl who developed acute onset Juvenile Rheumatoid Arthritis within 24 hours of her last injection of the Gardasil™ series contacted SANE Vax Inc. looking for more information.
In an effort to help her now very sick daughter the mother went to an MD practicing naturopath who conducted a toxicity test that eventually found HPV DNA in the girl’s blood. The significance of this finding is that it is highly unusual to find HPV DNA in the blood. HPV, if present in the body, exists in the epithelial (skin and mucosa) membranes. HPV or its DNA, by itself does not survive for any great length of time in the bloodstream. Why was the HPV DNA in her bloodstream two years post-vaccination?
Natural vs. Recombinant DNA
According to Dr. Lee, “‘Natural HPV DNA does not remain in the bloodstream for very long. However, the HPV DNA in Gardasil™ is not ‘natural’ DNA. It is a recombinant HPV DNA (rDNA) – genetically engineered – to be inserted into yeast cells for VLP (virus-like-particle) protein production. rDNA is known to behave differently from natural DNA. It may enter a human cell, especially in an inflammatory lesion caused by the effects of the aluminum adjuvant, via poorly understood mechanisms.